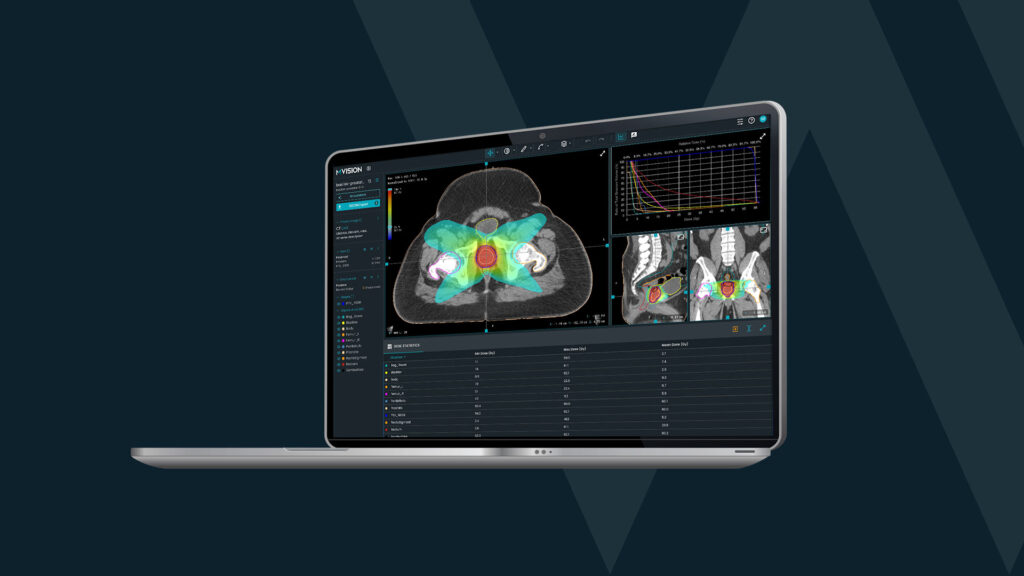
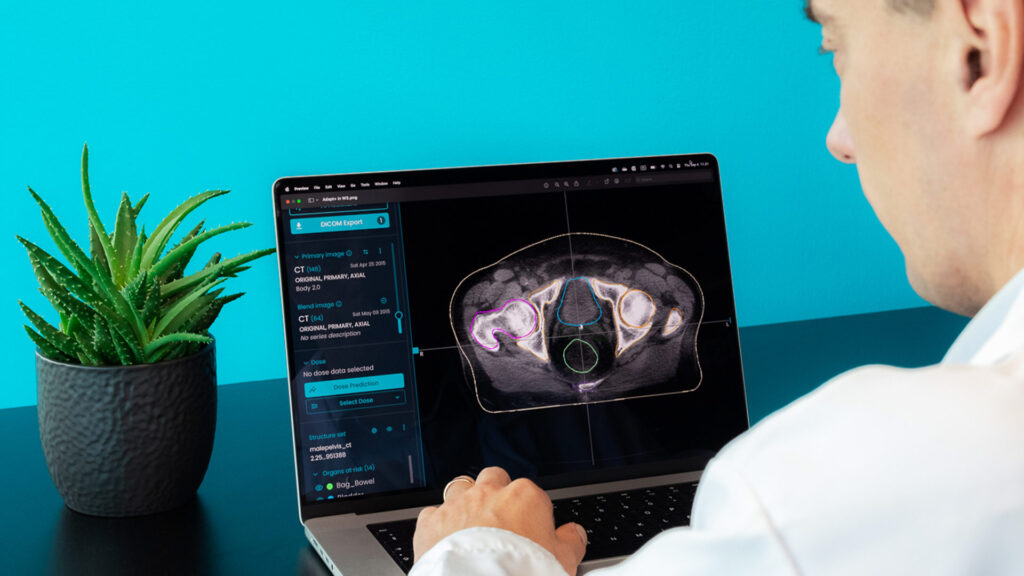
Time is money but it’s even more than that – time is life. Radiotherapy is one of the medical fields in which technological advances find a proper receptor. We find new ways to use outstanding computer capabilities to increase the quality of services and focus on the next steps to increase the quality of care. Artificial intelligence and machine-learning revolution reshape the world and bring new healthcare solutions. Auto-contouring for radiotherapy is the perfect example.
Below you will find brief answers to frequently asked questions regarding AI-based contouring solutions, in general. What are some common doubts related to this new “virtual assistant”?
- What is Artificial Intelligence (AI)?
- Is it helpful?
- Is it necessary?
- Is it better than humans?
- What are its limitations?
- Should we fully rely on it?
- Will we lose our skills?
- Are there any legal/regulatory aspects?
1.What is AI?
In a very simplified way, we can consider it a mechanism which can create a massive amount of connections between the elements of complex data. The deep learning systems are trained by using paired datasets, resulting in a high number of possible pathways. This way, it can give a more refined solution to a task which is not identical to what it had encountered before. It is “smarter” than the previous systems, which had a specific question connected to a certain answer. In radiotherapy, deep-learning based auto-contouring solutions are offering high quality predictions of the volumes that need to be targeted by radiation beams, or by contrary, to be protected.
2. Helpful? – Yes
The AI-based system generates the contours on the treatment planning scan. The time needed depends on the number of structures and the number of slices, but generally stays within a few minutes. After that, the contours are verified by a radiation oncologist and modified, if considered necessary.
According to multiple studies, using AI-based auto-contouring led to significant time savings compared to manual contouring and even using atlas-based contours (1). Manually editing the automatic contours was faster than exclusive manual contouring, because they usually need minimal adjustments (2-4). This situation happens only with quality predictions. Otherwise, correcting a poor quality structure takes longer than manually contouring it from the start. Generating a high number of accurate structures allows a better plan optimization, which is needed for high conformal treatments, the standard of care in modern radiotherapy.
3. Necessary? – Yes
We are facing a shortage of radiation oncologists, medical physicists and dosimetrists in many countries, and the gap between the offer and the needs is expected to grow (6). In parallel, the cancer burden is expected to rise, due to longer lifespan, increasing population and earlier diagnosis (7). In some regions, only one in 10 patients who need radiotherapy have access to it, but efforts are made to change this in the near future (8).
After the CT or MRI scanning for treatment planning, the volumes which have to be irradiated and those that need to receive the lowest possible irradiation dose are contoured. Based on that, the treatment planning is made and after review and optimisation, the treatment can start. The contouring is the most time-consuming part of the process and the interval from scanning to treatment is still longer than patients afford to wait. All available solutions, including AI-based contouring, need to be used to improve planning treatment procedures efficiency, quality, increase staff availability and decrease patient waiting time, without compromising overall treatment quality.
4. Better? – Depends
The quality of AI-based contours depends on the quality and consistency of the training data and the performance of the learning algorithm. It also depends on technical aspects, like the image quality of the datasets used for training and of the images on which it is applied to. It also depends on what we are comparing them to, what we consider to be as a reference or ground truth. Interestingly, when blindly evaluating manual or AI-generated contours, sometimes the AI-generated contours were preferred over the manual ones (5).
5. Reliable? – Yes
The variability of AI-based contours is lower than for human-made contours and the accuracy is more consistent. While evaluating if the AI-generated structures are clinically acceptable, in most of the studies there were no unacceptable contours. Most of them needed minor edits or not at all (2). However, being overconfident on the accuracy of the contours can make us less vigilant and miss the errors that could harm the patient, so we should always critically evaluate them.
6. Limitations? – Yes
AI models may show limitations in case of anatomical changes (tumor transformation or post-surgery), unusual patient positioning or image particularities (noise, heavy artifacts). For the Gross Target Volumes and Clinical Target Volumes there are clinical factors that have to be taken into account for a personalized treatment, so the AI based contours cannot offer a perfect prediction. A commissioning phase of such novel algorithms should be implemented to properly assess independent model performance on clinical datasets and all contours have to be verified, especially if they represent the targets or the organs at risk close to them.
7. Detrimental for education? – Not if properly used
There are concerns regarding the educational process of the trainees or continuous training of the seniors. A decreased need of manual contouring could impair the traditional learning process. However, detecting and fixing abnormalities in AI predictions require the same clinical knowledge as creating the structures from scratch. Alternative ways of training can replace or even surpass the quality of previous training style. Using AI and well curated contour sets, we can learn faster and better.
8. Legal? – Yes (but new regulations are emerging)
AI implementation is rapidly expanding, so creating a safe framework becomes a necessity. All the solutions for healthcare need to be verified and certified, so users should get informed and know what to search for and how to choose. Official regulations like the European Union AI act (9) and the White House Executive order on the Safe, Secure, and Trustworthy Development and Use of Artificial Intelligence (10) recognize the importance of responsible use of AI in healthcare, as one of the critical fields in our society. As a result, companies producing AI-based solutions need to stay in line with these regulations and requirements, which certifies both their quality and safety.
We will come back with more data and a detailed discussion on each of these aspects. Please feel free to add your comments and ask your own questions. We will be happy to answer.
References
- Gibbons E, Hoffmann M, Westhuyzen J, Hodgson A, Chick B, Last A. Clinical evaluation of deep learning and atlas-based auto-segmentation for critical organs at risk in radiation therapy. J Med Radiat Sci. 2023;70 Suppl 2(Suppl 2):15-25. doi:10.1002/jmrs.618
- Strolin S, Santoro M, Paolani G, et al. How smart is artificial intelligence in organs delineation? Testing a CE and FDA-approved Deep-Learning tool using multiple expert contours delineated on planning CT images. Front Oncol. 2023;13:1089807. Published 2023 Mar 2. doi:10.3389/fonc.2023.1089807
- Kiljunen T, Akram S, Niemelä J, et al. A Deep Learning-Based Automated CT Segmentation of Prostate Cancer Anatomy for Radiation Therapy Planning-A Retrospective Multicenter Study. Diagnostics (Basel). 2020;10(11):959. Published 2020 Nov 17. doi:10.3390/diagnostics10110959
- Turcas A, Leucuta D, Balan C, et al. Deep-learning magnetic resonance imaging-based automatic segmentation for organs-at-risk in the brain: Accuracy and impact on dose distribution. Phys Imaging Radiat Oncol. 2023;27:100454. Published 2023 Jun 6. doi:10.1016/j.phro.2023.100454
- Wu Y, Kang K, Han C, et al. A blind randomized validated convolutional neural network for auto-segmentation of clinical target volume in rectal cancer patients receiving neoadjuvant radiotherapy. Cancer Med. 2022;11(1):166-175. doi:10.1002/cam4.4441
- Limb M. Shortages of radiology and oncology staff putting cancer patients at risk, college warns. BMJ. 2022;377:o1430. Published 2022 Jun 10. doi:10.1136/bmj.o1430
- Joint Research Center. European Cancer Information System: 21% increase in new cancer cases by 2040. Acceses on 20 October 2023. https://joint-research-centre.ec.europa.eu/jrc-news-and-updates/european-cancer-information-system-21-increase-new-cancer-cases-2040-2022-03-16_en
- Rays of Hope: Widening Global Access to Cancer Care. Accessed on 20 October 2023. https://www.iaea.org/newscenter/news/rays-of-hope-widening-global-access-to-cancer-care
- EU AI Act: first regulation on artificial intelligence. Accessed on 15 November 2023. https://www.europarl.europa.eu/news/en/headlines/society/20230601STO93804/eu-ai-act-first-regulation-on-artificial-intelligence
- Executive Order on the Safe, Secure, and Trustworthy Development and Use of Artificial Intelligence. Accessed on 15 November 2023. https://www.whitehouse.gov/briefing-room/presidential-actions/2023/10/30/executive-order-on-the-safe-secure-and-trustworthy-development-and-use-of-artificial-intelligence/