Helsinki, September 2025 – MVision AI is proud to announce that Dose+, its AI-powered solution for dose prediction and personalised radiotherapy treatment planning, has received FDA 510(k) clearance. This authorises clinical use across the United States, marking an important milestone for MVision AI and expanding access to advanced planning tools for U.S. clinicians.
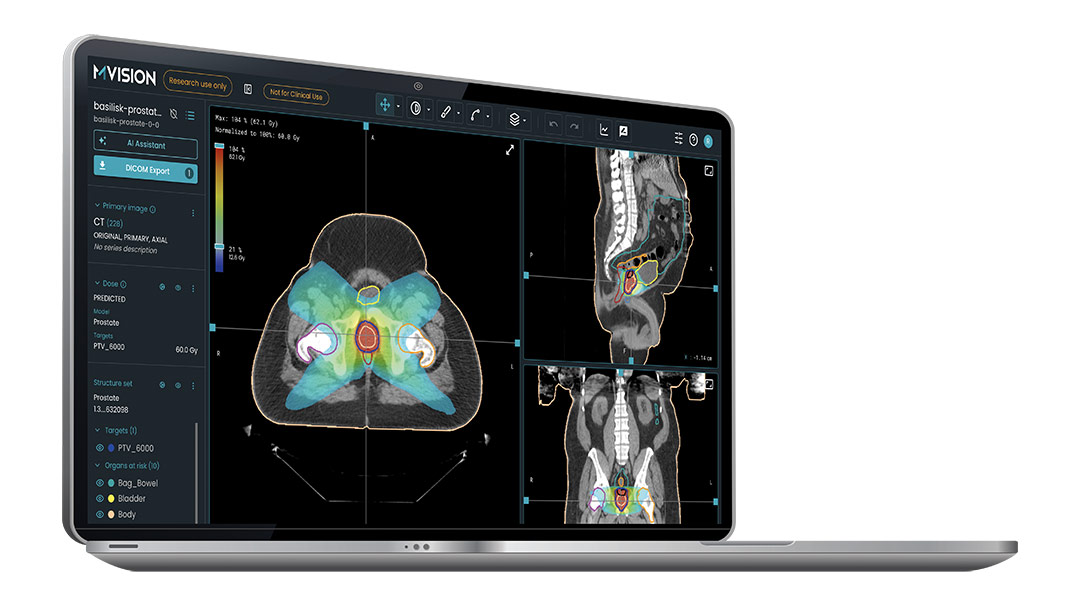
By analysing individual anatomy, Dose+ generates tailored 3D dose estimates that align with clinical best practices. The system produces clinically achievable VMAT-style dose distributions directly from CT images and structure sets using a simple DICOM transfer. These 3D dose distributions can be imported into any DICOM-compliant treatment planning system, giving clinicians a reliable starting point for plan refinement.
Boost in planning consistency
Reduced manual workload
Dose+ delivers measurable benefits: it boosts planning consistency by up to 20%, reduces manual workload by up to 70%, achieves a clinical acceptance rate above 90%, and produces predictions in an average of just 2 minutes.
“Achieving FDA 510(k) clearance for Dose+ is a monumental achievement for MVision AI,” said Mahmudul Hasan, Founder and Executive Director of MVision AI. “This clearance opens the door for U.S. clinicians to use AI-powered dose prediction tools that improve efficiency, consistency, and quality in radiation therapy planning.”
The clearance comes at a critical time. The American Cancer Society projects more than 2 million new cancer cases in the United States in 2025 (1). It is estimated that about half of all people diagnosed with cancer will undergo radiation therapy during their treatment (2). As a result, solutions like Dose+ can help clinical teams manage growing workloads while maintaining high-quality care.
“With this U.S. market entry, MVision AI is expanding the clinical availability of its AI-powered planning ecosystem—including Contour+, Guide, Verify, and now FDA-cleared Dose+,” added Hasan. “Our goal is to give radiation oncology professionals intelligent automation that strengthens workflows and supports more patient-focused care.”
MVision AI continues to drive innovation in radiation therapy with a suite of AI-powered solutions, advancing its mission to streamline workflows, enhance treatment quality, and make care more consistent and patient-centered worldwide.
For more information, visit www.mvision.ai or contact info@mvision.ai.
About MVision AI
MVision AI is a leading provider of AI-powered solutions for radiation therapy treatment planning. Our mission is to empower radiation oncology professionals with cutting-edge technology to streamline workflows, enhance treatment quality, and facilitate more patient-focused care. With a commitment to innovation and excellence, MVision AI continues to advance cancer care through the power of AI.
References
- American Cancer Society. Cancer Facts & Figures 2025. Atlanta: American Cancer Society; 2025. Available from: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2025/2025-cancer-facts-and-figures-acs.pdf
- Advanced Medical Technology Association (AdvaMed). Radiation Therapy. Washington, DC: AdvaMed; 2024. Available from: https://www.advamed.org/our-work/sectors/radiation-therapy/

