Key facts:
- Professionals working in 40 clinics from 31 countries assessed MVision AI’s Contour+ solution (Figure 1).
- More than 11 800 structures were evaluated on 1353 scans (Figure 2).
- Detailed results can be found in 5 full text articles published in 3 Peer-reviewed journals (Diagnostics 2020, Physics and Imaging in Radiation Oncology 2022 and 2023, Frontiers in Oncology 2023).
- Brief data are included in 12 posters or oral presentations from international meetings (IAEA ICARO-3 2021, DEGRO 2022, SEOR 2022, ESTRO 2023, AAPM 2023, Corso di laurea in fisica medicale, 2023) (Table 1).
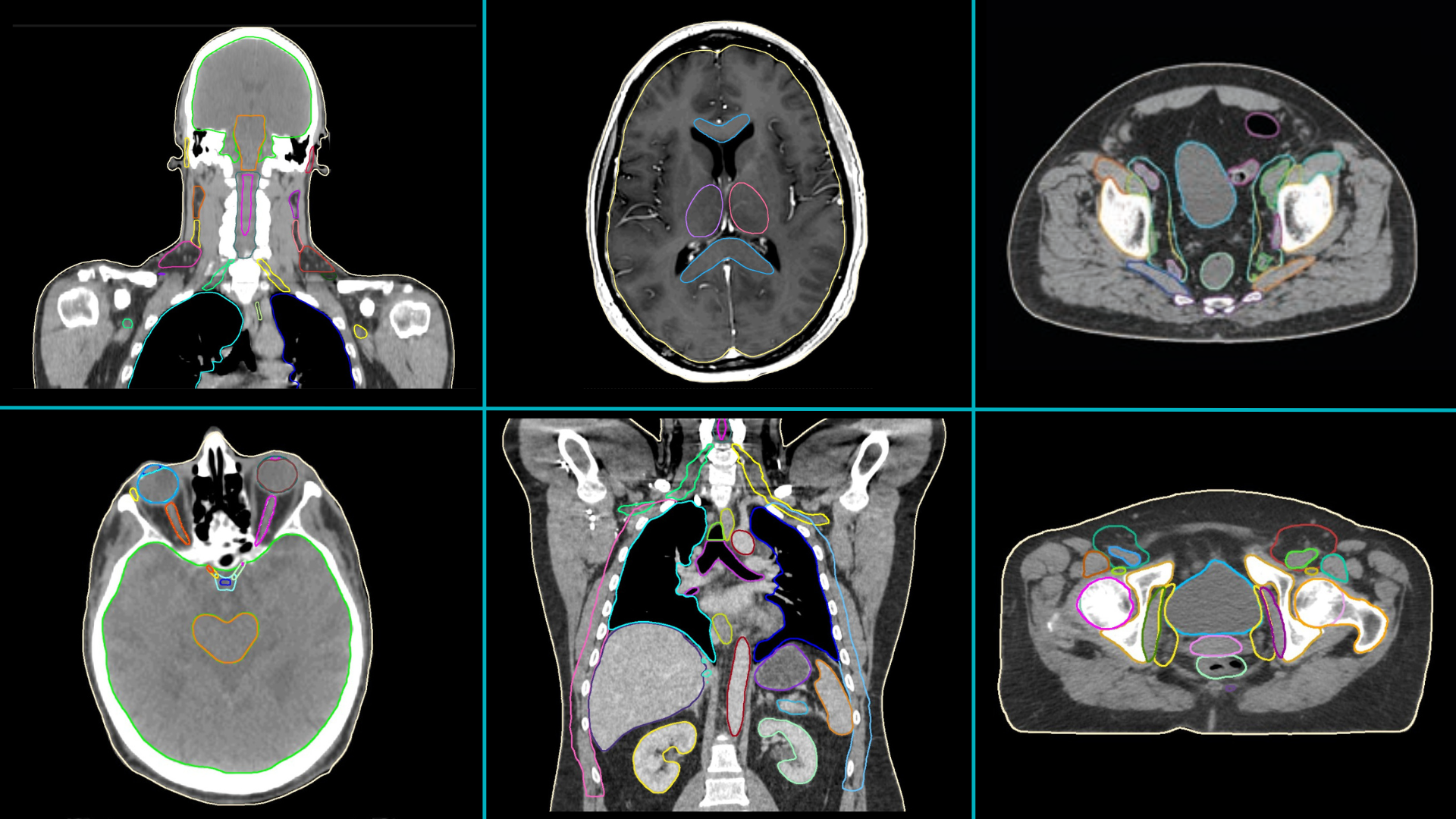
- Anatomical sites for which MVision AI models have been used for automatic contouring in the research projects (and the number of studies including them) are: male pelvis (9), breast (4), head and neck (4), thorax (3), female pelvis (3), brain (2), abdomen (2), heart (2), jaws (1) (Figure 3).
- The studies evaluated MVision AI contours’ accuracy (14), time save (10), impact on interobserver variability (4), impact on dose (3) (Figure 4).




Table 1. Currently published/presented research projects that involved MVision AI’s Contour+ solution
Notes: *Articles in German. **Most of the data are common in the full text article and the poster.
Abbreviations: NA – Not available in the published text; IAEA-ICARO-3 – International Atomic Energy Agency – International Conference on advances in Radiation Oncology; SEOR – Spanish Radiation Oncology Society; DEGRO – German Radiation Oncology Society; ESTRO – European Society for Therapeutic Radiology and Oncology; AAPM – American Associations of Physicists in Medicine; OARs – organs at risk; CE – European certification for safety, health and environmental protection requirements; FDA – Food and Drug Administration; CT – Computed Tomography; MRI – Magnetic Resonance Imaging; AI – Artificial Intelligence; RIVA – ramus intervetricularis anterior.
Excerpts from the scientific reports:
- Using a general deep learning-based automated segmentation tool*** for CT images saves time and improves consistency [1].
- Using small single-institutional radiotherapy datasets with consistently-defined rectal volumes when training auto segmentation algorithms created contours of similar quality as the same algorithm trained on large multi institutional datasets [2].
- The application of the deep learning tool*** resulted satisfactory, especially in complex delineation cases, improving the radiation oncologists’ inter-agreement of delineated volumes of interest and saving time [3].
- Auto-segmentation*** was significantly faster, with minor differences in dose distribution caused by geometric variations [4].
- MVision offered 143 CT contours with a median vDSCs 0.88 and substantial time savings: on average 43.6 minutes across all structures [5].
- Contouring time is reduced when using auto-contouring solutions. The structure definition in the training set is important [6].
- MVision significantly contributed to reducing contouring time, enhancing patient care by shortening their waiting times [7].
- AI-based*** target volumes have a great resemblance to the original ones. As part of the Irradiation of localized prostate cancer, autocontouring is promising and provides a time-saving approach to target volume definition [8].
- The AI-based contours*** show great resemblance both in the total volumes and in the DSC with the original medical contours [9].
- The developed AI-based model*** of the jawbones is precise, time-efficient and shows good results in CTs with and without metal artifacts. The new jaw model presents a practical and economical option for radiation oncologists and oral and maxillofacial surgeons is the radiation dose in implant-relevant areas potentially reducing jaw segments [10].
- An AI-based auto-contouring algorithm*** for cardiac substructures enable time-effective creation of contours for right ventricle, left ventricle, right atrium and left atrium in the context of thoracic radiation planning. The use of the algorithm enables retrospective and prospective analyses of the dose load of cardiac substructures in the context of thoracic radiation and enables a user-independent, automated contour creation [11].
- AI-based contouring*** leads to more accurate and better reproducible contouring of organs at risk [12].
- Use of AI volumes*** for breast patients requiring nodal treatment has streamlined the existing Breast/Chest wall ±axilla and supraclavicular fossa carepath, and accelerated VMAT roll-out for all high-risk breast patients, with anticipated toxicity reduction. An increase in confidence for dosimetrists in nodal plan optimization has reduced routine tasks requiring clinician input [13].
- AI-assisted contouring*** significantly reduced inter observer variability across multiple institutions located in low and middle income countries globally. The results add a significant piece of evidence favoring further adoption of AI-assisted contouring worldwide [14].
- AI-based segmentation*** is significantly faster than manual delineation. The geometric accuracy is high for large structures, which are also the most laborious to manually delineate [15].
- The auto-segmentation models*** demonstrated superior accuracy and a high degree of consistency, offering the potential for significant time saving in contour delineation [16].
- Deep learning-based auto-contouring*** outperformed STAPLE and random forest algorithms in cervical cancer, especially in rectum and bladder. (…) The strong time reduction could suggest an easier deep learning algorithm introduction in radiotherapy workflow, thanks also to the possibility to automate it [17].
Notes: the number in brackets refers to the corresponding article in the table. ***general terms referring to the evaluated solution, provided by MVision AI
Abbreviations: vDSC – volumetric Dice Similarity Coefficient – a measure of the similarity between the automated contour and the reference contour; VMAT – volumetric modulated arc therapy; STAPLE – Simultaneous Truth And Performance Level Evaluation.