Since 2022, when World Cancer’s Day “Close the Care Gap” campaign was launched, we have probably all heard this motto. Disparities in cancer care, unfortunately, represent day to day realities and enforce the need of smart solutions for an efficient use of the resources. Currently, about 2 million people are dying of cancer each year in low and middle income countries [1]. The cancer burden is expected to increase in these areas in the future, due to rising population, longer life expectancy and lifestyle changes. By 2030, three quarters of the cancer deaths are estimated to occur in these parts of the world [2]. Many lives could be spared if radiotherapy would be more accessible, so combined efforts are made for improving cancer care.
Applying deep-learning based solutions for auto-contouring predictions was previously shown to decrease inter-observer variability and delineation time [3] [4] [5]. This creates the possibility of harmonizing the clinical practice and reducing the interval from the planning scan to the treatment start. Most of the current data come from high-income countries, but the limited resources environment would benefit even more from the implementation of a contouring supporting tool which would translate into reducing the diagnosis-to-treatment interval.
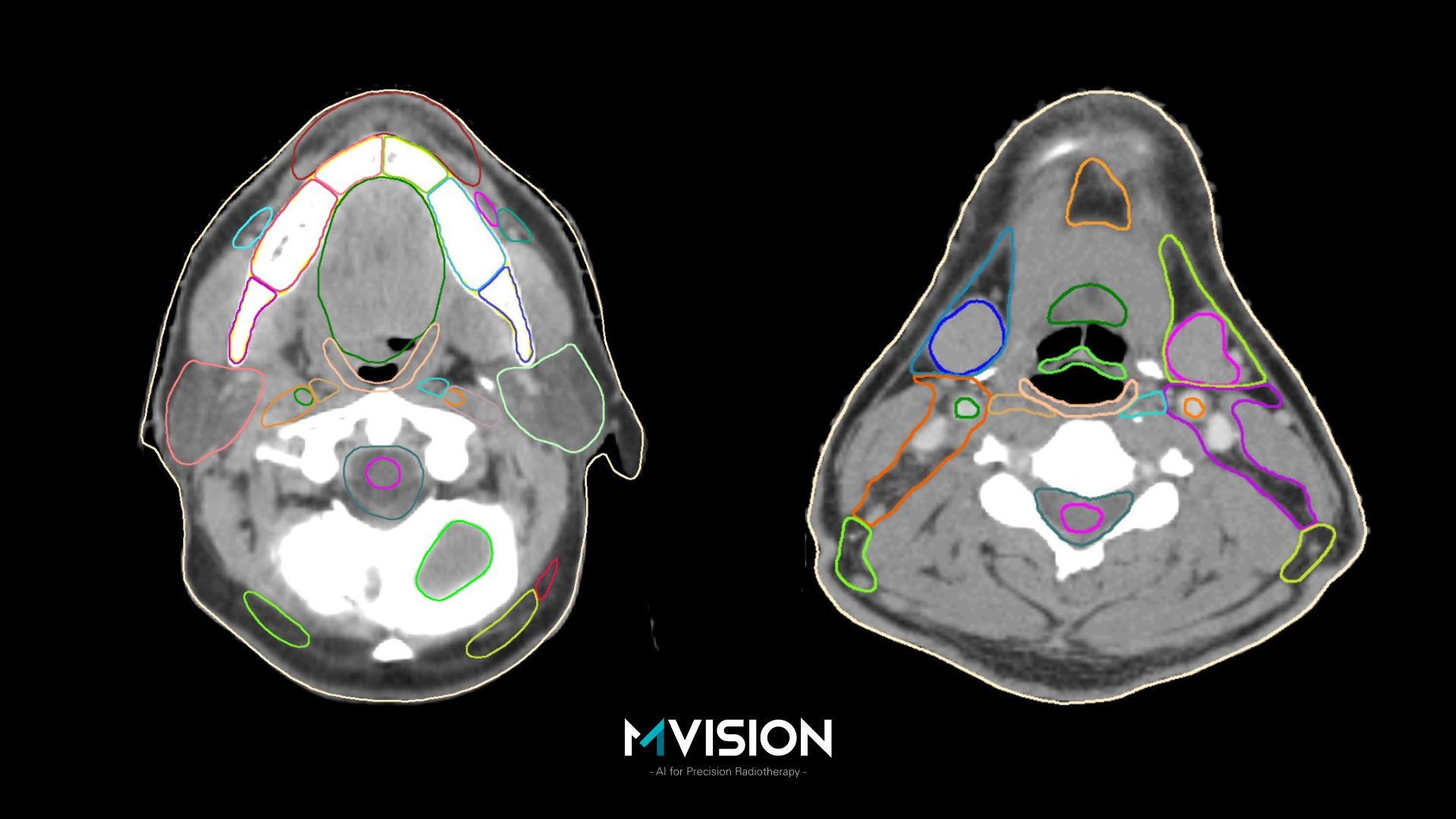
An international multi-centric randomized trial evaluated the impact of E-learning interventions on the organs at risk contouring for head and neck cancers. The ELAISA project (the Potential of E-Learning interventions for AI-assisted contouring Skills in rAdiotherapy) resulted from the partnership of the Aarhus University Hospital and the International Atomic Energy Agency (IAEA). E-learning was made possible by the support of MVision AI and EduCase.
Mathis Ersted Rasmussen presented the first results of the trial at ESTRO 2023 during the Highlights of Proffered Papers – Latest clinical trials session. The data came from 89 participants located in 22 low and middle income countries, situated on all 5 continents. The following structures were delineated: brainstem, spinal cord, parotid gland, submandibular gland, thyroid, oral cavity, optic nerve and mandible. One group manually delineated the structures (the control group) and the other group used AI-assisted delineation (the intervention group). Interobserver variation was analyzed by comparing the contour of each participant with the median of the group’s contours. The contouring bias was assessed by comparing each contour to a reference structure, which resulted from the consensus of three experts, and reviewed by an external consultant. Contouring time was automatically recorded within the platform. Participants were instructed to create clinically acceptable contours, using the same guidelines as used for developing MVision AI’s Contour+ Head and Neck model.
When compared with the manual contour group, the AI-assisted group had lower interobserver variation for all the 8 structures, lower contouring bias for 5 structures and lower contouring time for 6 structures. It should be mentioned that there were four geographical misses, and all were in the manual contouring group [6].
This important study is a proof of concept, but the results are also indirectly speaking about the quality of the MVision GBS™ AI-based contouring solution, which proved to be useful also in a limited resources environment.
Health professionals’ training represents a fundamental condition for delivering high quality care, and the contouring skills are essential for preparing a precise and personalized radiotherapy plan. MVision AI is offering a fast, reliable and efficient solution for improving cancer care, bringing its contribution to closing the care gap.
References
[1] https://gco.iarc.fr/today/data/factsheets/populations/988-low-middle-income-fact-sheets.pdf
[2] Pramesh CS, Badwe RA, Bhoo-Pathy N, et al. Priorities for cancer research in low- and middle-income countries: a global perspective. Nat Med. 2022;28(4):649-657. doi:10.1038/s41591-022-01738-x
[3] Turcas A, Leucuta D, Balan C, et al. Deep-learning magnetic resonance imaging-based automatic segmentation for organs-at-risk in the brain: Accuracy and impact on dose distribution. Phys Imaging Radiat Oncol. 2023;27:100454. Published 2023 Jun 6. doi:10.1016/j.phro.2023.100454
[4] Strolin S, Santoro M, Paolani G, et al. How smart is artificial intelligence in organ delineation? Testing a CE and FDA-approved Deep-Learning tool using multiple expert contours delineated on planning CT images. Front Oncol. 2023;13:1089807. Published 2023 Mar 2. doi:10.3389/fonc.2023.1089807
[5] Kiljunen T, Akram S, Niemelä J, et al. A Deep Learning-Based Automated CT Segmentation of Prostate Cancer Anatomy for Radiation Therapy Planning-A Retrospective Multicenter Study. Diagnostics (Basel). 2020;10(11):959. Published 2020 Nov 17. doi:10.3390/diagnostics10110959
[6]https://www.estro.org/Congresses/ESTRO-2023/1438/highlightsofprofferedpapers-latestclinicaltrials/13338/benefitofai-assistedorgan-at-riskcontouringinhead-